A new lithium-air battery that manages to multiply by four the capacity of lithium batteries and exceed 1,000 cycles
A new lithium-air battery that manages to multiply by four the capacity of lithium batteries and exceed 1,000 cycles
The dream of achieving a battery that allows an electric car to offer the autonomy of a model with a combustion engine, without having to ballast the vehicle with tons is getting closer. One of the latest movements comes from the Illinois Institute of Technology and the Argonne National Laboratory, dependent on the United States Department of Energy, which have developed a lithium-air battery that could make that dream come true.
It is energy, and volumetric density is important in applications such as road transport. But the key is in other potentially interesting sectors such as air. Something that the new lithium-air design developed by these teams could make it possible to begin to address it more broadly.
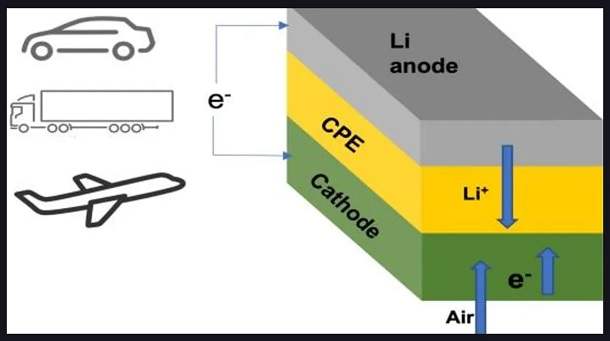
A battery has three main components: the anode (negative terminal), the cathode (positive terminal), and the electrolyte. The electrolyte is a substance that helps ions (charged particles) move between the anode and the cathode, allowing the battery to produce electrical energy.
In a traditional lithium-air battery, the electrolyte is a liquid substance that can be prone to leakage, evaporation, and other issues that can affect the battery’s performance and safety. However, in this new design, scientists have replaced the liquid electrolyte with a solid electrolyte, which is more stable and less prone to problems.
The solid electrolyte used in this design helps to prevent the formation of harmful byproducts that can degrade the battery’s performance over time. It also allows for higher energy densities, which means that the battery can store more energy per unit of weight or volume.
This new lithium-air design with a solid electrolyte represents an important advance in battery technology, with the potential to improve efficiency, durability, and safety of lithium-air batteries for a wide range of applications.
Batteries with greater thermal stability are safer because they are less likely to overheat or catch fire. That is particularly important for batteries used in cars, which can generate a lot of heat and need to be able to operate in a wide range of temperatures. If a battery gets too hot, it can cause a dangerous situation for the people in the vehicle and for the vehicle itself.
For example, batteries with better thermal stability can help to reduce the risk of fires and improve overall safety in electric cars. In addition, they can help to extend the range of the car, because they can operate more efficiently at higher temperatures.
Lithium-air battery, greater safety, and energy density
Energy density is a measure of how much energy a battery can store per unit of weight or volume. A higher energy density means that a battery can store more energy in a smaller space, which translates into longer battery life and greater autonomy.
The chemistry used in the batteries developed by this team can increase energy density up to four times compared to current lithium batteries. That means that the batteries can store more energy in a smaller and lighter package, allowing devices to run for longer periods without needing to be recharged.
For example, a smartphone with a battery that has four times the energy density could last four times longer on a single charge than a phone with a current lithium battery. That would be a significant improvement in the user experience, as it would allow people to use their devices for longer periods without worrying about running out of power.
The increased energy density of these batteries represents a breakthrough in battery technology, with the potential to revolutionize a wide range of applications, from portable electronics to electric vehicles and grid-scale energy storage.
The designers of this lithium-air battery claim that it has the highest projected energy density of any battery technology being considered for the next generation beyond lithium-ion.
Energy density is a measure of how much energy a battery can store per unit of weight or volume. A battery with a higher energy density can store more energy in a smaller space, which means it can provide more power for a longer period without needing to be recharged.
The lithium-ion battery is currently the most common type of battery used in many applications, including smartphones, laptops, and electric vehicles. However, it has limitations in terms of its energy density and safety.
The designers of the lithium-air battery claim that their battery technology can offer a higher energy density than any other battery technology currently being considered for the next generation beyond lithium-ion. That means that it has the potential to provide more power for a longer period without needing to be recharged, making it a promising technology for a wide range of applications.
This claim suggests that the lithium-air battery could be a game-changing technology with the potential to revolutionize battery technology and transform many industries that rely on batteries for power.
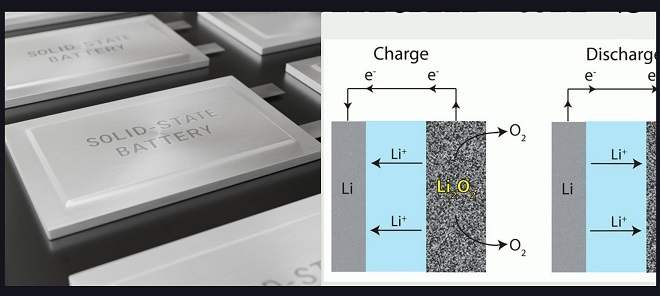
In earlier lithium-air battery designs, the battery worked by combining lithium from the anode (negative terminal) with oxygen from the air during discharge. This process produced a compound called lithium peroxide or superoxide in the cathode (positive terminal) of the battery.
During charging, the lithium peroxide or superoxide decomposed back into its lithium and oxygen components, which could be used again to generate electricity when needed. This chemical sequence of combining and then separating lithium and oxygen in the battery allows it to store and release energy on demand.
A liquid electrolyte was used to help the lithium ions move from the anode to the cathode during discharge and back again during charging. However, this liquid electrolyte could be unstable and lead to safety issues in some cases.
In newer lithium-air battery designs, a solid electrolyte is used instead of a liquid one. That improves the stability and safety of the battery while still allowing it to store and release energy on demand through the chemical sequence of combining and separating lithium and oxygen.
A solid electrolyte made of a ceramic polymer material composed of relatively inexpensive elements in the form of nanoparticles has been used in this new lithium-air battery design.
This solid electrolyte allows the battery to undergo chemical reactions that produce lithium oxide (Li2O) during discharge. Lithium oxide is a compound made up of lithium and oxygen, and it stores the energy generated by the battery during discharge.
The use of a solid electrolyte is a significant improvement over previous designs that used liquid electrolytes. A solid electrolyte is safer and more stable than a liquid one and does not evaporate or leak as easily. That makes the battery more reliable and easier to use.
Furthermore, the use of relatively inexpensive elements in the form of nanoparticles makes the battery more affordable and accessible, potentially opening up new applications for the technology.
This new lithium-air battery design represents an exciting breakthrough in battery technology with the potential to provide higher energy density and greater safety than previous designs.
Rachid Amine, a researcher at the Argonne Laboratory, explains that the energy density of a battery is directly related to the number of stored electrons it contains. More stored electrons mean a higher energy density.
In the case of a lithium-air battery, the chemical reaction that occurs during discharge can either produce lithium superoxide, peroxide, or lithium oxide. The former only involves one or two stored electrons per oxygen molecule, while the latter involves four electrons.
That means that the chemical reaction that produces lithium oxide has the potential to store more energy per oxygen molecule than the reaction that produces lithium superoxide or peroxide. As a result, a lithium-air battery that produces lithium oxide has the potential to offer a higher energy density than one that produces lithium superoxide or peroxide.
That suggests that the new solid electrolyte used in the lithium-air battery design mentioned earlier, which facilitates the production of lithium oxide during discharge, has the potential to significantly increase the energy density of the battery making it a promising technology for a wide range of applications.
The team’s lithium-air battery design represents a significant breakthrough in battery technology, as it is the first lithium-air battery to achieve a four-electron reaction at room temperature.
The four-electron reaction involves the production of lithium oxide, which can store more energy per oxygen molecule than other reactions that produce lithium superoxide or peroxide. That means that the new solid electrolyte used in this design has the potential to significantly increase the energy density of the battery.
The battery is designed to work with oxygen supplied by air from the surrounding environment. That is significant because it eliminates the need for oxygen tanks to operate the battery, which was a problem with earlier designs. That makes the battery more practical and easier to use in a variety of applications.
This new lithium-air battery design represents a significant step forward in battery technology, with the potential to offer higher energy density, greater safety, and more practical operation than previous designs.
One of the challenges with lithium-air batteries has been their low shelf life, or the length of time they can maintain their energy storage capacity when not in use. However, the developers of the new solid electrolyte lithium-air battery design mentioned earlier have overcome this challenge and demonstrated the battery’s stability through more than 1,000 repeated charge and discharge cycles in their first prototypes.
This breakthrough has allowed the designers to set a goal of achieving a battery with an energy density of 1,200 Wh/kg. That is almost four times better than the energy density of current commercial lithium batteries, which typically have energy densities of around 300 Wh/kg.
This increased energy density could have significant implications for a range of industries and applications, from electric vehicles to renewable energy storage. Overall, the new solid electrolyte lithium-air battery design holds great promise for the future of battery technology.
While the new solid electrolyte lithium-air battery design holds great promise, it’s important to note that there are currently no specific dates for its arrival in the commercial market. However, given the pace of development in the battery industry, it’s reasonable to expect that more than 10 years of work on this type of chemical will yield results shortly.
Some industry experts speculate that the first packs of these batteries could begin to be installed by 2025. However, it’s important to keep in mind that battery technology is complex, and there may be additional hurdles to overcome before these batteries become widely available.
Nonetheless, the potential benefits of this new technology, including greater energy density, increased safety, and more practical operation, make it an exciting development to watch in the coming years.
Related Post



